Alison K. Criss
- Website: http://mic.med.virginia.edu/criss/
Primary Appointment
Professor, Microbiology, Immunology, and Cancer Biology
Education
- BA, Biology and Chemistry, Williams College
- PhD, Cell and Developmental Biology, Harvard University
- Postdoc, Microbiology, Northwestern University
Research Disciplines
Cell and Developmental Biology, Immunology, Infectious Diseases/Biodefense, Microbiology
Research Interests
Cellular and molecular mechanisms of Neisserial pathogenesis
Research Description
Since N. gonorrhoeae has no niche outside of humans, the biology of this organism is exquisitely tailored to life in the human urogenital tract. Not only is N. gonorrhoeae able to exploit the nutritional and environmental conditions at this site, but the bacterium also has an extraordinary ability to thwart challenges by the host immune response. N. gonorrhoeae evades humoral immune recognition by undergoing extensive variation of its surface structures, including high-frequency sequence changes in the N. gonorrhoeae pilin gene that lead to antigenic variation of the type IV pilus. N. gonorrhoeae is also highly adept at surviving confrontations with the innate immune system. Acute gonorrhea is a highly inflammatory disease characterized by the production of an exudate containing large numbers of neutrophils. Although neutrophils are the body's first defenders against bacterial and fungal challenge, neutrophils are ineffective at killing N. gonorrhoeae, and gonorrheal exudates contain viable, infectious bacteria.
Our research is currently centered on identifying how N. gonorrhoeae resists neutrophil clearance. We are addressing how neutrophils recognize N. gonorrhoeae, which antimicrobial activities neutrophils direct against N. gonorrhoeae, and how N. gonorrhoeae withstands or thwarts these activities. We use a combination of cell biology, molecular biology, bacterial genetics and biochemistry to address these questions. The insights gleaned from these studies will help us to understand in general how the mucosal innate immune system defends against infection and how pathogens exploit mucosal defenses to aid in colonization and transmission.
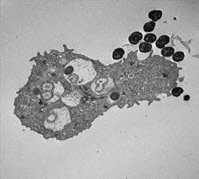
Thin-section transmission electron micrograph of N. gonorrhoeae-infected neutrophil.
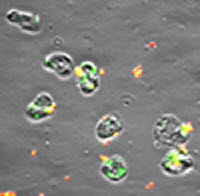
Fluorescence/phase-contrast image of human neutrophils infected with strain FA1090 N. gonorrhoeae for 60 minutes. Green particles are intracellular bacteria; red/yellow particles are extracellular bacteria.
Personal Statement
Since N. gonorrhoeae has no niche outside of humans, the biology of this organism is exquisitely tailored to life in the human urogenital tract. Not only is N. gonorrhoeae able to exploit the nutritional and environmental conditions at this site, but the bacterium also has an extraordinary ability to thwart challenges by the host immune response. N. gonorrhoeae evades humoral immune recognition by undergoing extensive variation of its surface structures, including high-frequency sequence changes in the N. gonorrhoeae pilin gene that lead to antigenic variation of the type IV pilus. N. gonorrhoeae is also highly adept at surviving confrontations with the innate immune system. Acute gonorrhea is a highly inflammatory disease characterized by the production of an exudate containing large numbers of neutrophils. Although neutrophils are the body's first defenders against bacterial and fungal challenge, neutrophils are ineffective at killing N. gonorrhoeae, and gonorrheal exudates contain viable, infectious bacteria.
Our research is currently centered on identifying how N. gonorrhoeae resists clearance by the human innate immune system. We are addressing how neutrophils recognize N. gonorrhoeae, which antimicrobial activities neutrophils direct against N. gonorrhoeae, and how N. gonorrhoeae withstands or thwarts these activities. We use a combination of cell biology, molecular biology, bacterial genetics and biochemistry to address these questions. The insights gleaned from these studies will help us to understand in general how the mucosal innate immune system defends against infection and how pathogens exploit mucosal defenses to aid in colonization and transmission. Specific areas of interest are outlined below.
Resistance of neutrophils to non-oxidative neutrophil killing: We have found that a significant proportion of N. gonorrhoeae presented to primary human neutrophils in vitro survive early infection and grow in association with them over time. Neutrophils generate reactive oxygen species (ROS) such as hydrogen peroxide and hypochlorous acid as part of their antimicrobial arsenal, but inhibition of neutrophil ROS production has no effect on N. gonorrhoeae survival. In the course of these studies, we identified bacterial mutants that are more sensitive to non-oxidative neutrophil killing. We plan to examine which non-oxidative neutrophil factors are active against N. gonorrhoeae and the reasons for the susceptibility of our bacterial mutants to non-oxidative neutrophil killing. We have also gained evidence that neutrophil phagosomes contain viable N. gonorrhoeae. We plan to characterize the neutrophil subcellular compartment(s) in which N. gonorrhoeae are found and examine their maturation over time.
N. gonorrhoeae suppression of neutrophil ROS production: In examining why neutrophil oxidative mechanisms are dispensable for killing of N. gonorrhoeae, we made the surprising observation that neutrophils generate little to no ROS after infection with exponentially-growing N. gonorrhoeae. Moreover, N. gonorrhoeae infection suppresses the ability of neutrophils to generate ROS in response to a variety of stimuli, including S. aureus and formylated peptides. We hypothesize that live N. gonorrhoeae produce factors that interfere with signaling pathways that normally lead to neutrophil ROS production. We are examining how N. gonorrhoeae impede neutrophil signal transduction and which bacterial gene products are responsible for this effect.

Thin-section transmission electron micrograph of N. gonorrhoeae-infected neutrophil.  N. gonorrhoeae for 60 minutes. Green particles are intracellular bacteria; red/yellow particles are extracellular bacteria.
N. gonorrhoeae for 60 minutes. Green particles are intracellular bacteria; red/yellow particles are extracellular bacteria.
Training
- Biodefense & Infectious Diseases Short-Term Training to Increase Diversity in Biomedical Sciences
- Global Biothreats Training Program
- Infectious Diseases Training Program
- Interdisciplinary Training Program in Immunology
- Training in Cell and Molecular Biology
- Training in the Pharmacological Sciences
Selected Publications
2024
Cardenas, A. J., Thomas, K. S., Broden, M. W., Ferraro, N. J., Pires, M. M., John, C. M., . . . Criss, A. K. (2024). Neisseria gonorrhoeae scavenges host sialic acid for Siglec-mediated, complement-independent suppression of neutrophil activation. MBIO, 15(5). doi:10.1128/mbio.00119-24
Cardenas, A. J., Thomas, K. S., Broden, M. W., Ferraro, N. J., John, C. M., Pires, M. M., . . . Criss, A. K. (2024). Neisseria gonorrhoeae scavenges host sialic acid for Siglec-mediated, complement-independent suppression of neutrophil activation.. bioRxiv. doi:10.1101/2024.01.17.576097
2023
Geslewitz, W. E., Cardenas, A., Zhou, X., Zhang, Y., Criss, A. K., & Seifert, H. S. (2024). Development and implementation of a Type I-C CRISPR-based programmable repression system for Neisseria gonorrhoeae. MBIO, 15(2). doi:10.1128/mbio.03025-23
Gray, M. C., Thomas, K. S., Lamb, E. R., Werner, L. M., Connolly, K. L., Jerse, A. E., & Criss, A. K. (2023). Evaluating vaccine-elicited antibody activities against Neisseria gonorrhoeae: cross-protective responses elicited by the 4CMenB meningococcal vaccine. INFECTION AND IMMUNITY. doi:10.1128/iai.00309-23
Werner, L. M., & Criss, A. K. (2023). Diverse Functions of C4b-Binding Protein in Health and Disease. JOURNAL OF IMMUNOLOGY, 211(10), 1443-1449. doi:10.4049/jimmunol.2300333
Potter, A. D., Baiocco, C. M., Papin, J. A., & Criss, A. K. (2023). Transcriptome-guided metabolic network analysis reveals rearrangements of carbon flux distribution in Neisseria gonorrhoeae during neutrophil co-culture. MSYSTEMS. doi:10.1128/msystems.01265-22
Potter, A. D., & Criss, A. K. (2024). Dinner date: Neisseria gonorrhoeae central carbon metabolism and pathogenesis. EMERGING TOPICS IN LIFE SCIENCES, 8(1), 15-28. doi:10.1042/ETLS20220111
Smirnov, A., Daily, K. P., Gray, M. C., Ragland, S. A., Werner, L. M., Johnson, M. B., . . . Criss, A. K. (2023). Phagocytosis via complement receptor 3 enables microbes to evade killing by neutrophils. JOURNAL OF LEUKOCYTE BIOLOGY, 114(1), 1-20. doi:10.1093/jleuko/qiad028
Werner, L. M. M., Alcott, A., Mohlin, F., Ray, J. C. C., Dufrisne, M. B., Smirnov, A., . . . Criss, A. K. K. (2023). Neisseria gonorrhoeae co-opts C4b-binding protein to enhance complement-independent survival from neutrophils. PLOS PATHOGENS, 19(3). doi:10.1371/journal.ppat.1011055
Liyayi, I. K., Forehand, A. L., Ray, J. C., & Criss, A. K. (2023). Metal piracy by Neisseria gonorrhoeae to overcome human nutritional immunity. PLOS PATHOGENS, 19(2). doi:10.1371/journal.ppat.1011091
John, C. M., Phillips, N. J., Cardenas, A. J., Criss, A. K., & Jarvis, G. A. (2023). Comparison of lipooligosaccharides from human challenge strains of Neisseria gonorrhoeae. FRONTIERS IN MICROBIOLOGY, 14. doi:10.3389/fmicb.2023.1215946
2022
Crawford, M. A., Ward, A. E., Gray, V., Bailer, P., Fisher, D. J., Kubicka, E., . . . Hughes, M. A. (2022). Disparate Regions of the Human Chemokine CXCL10 Exhibit Broad-Spectrum Antimicrobial Activity against Biodefense and Antibiotic-Resistant Bacterial Pathogens. ACS INFECTIOUS DISEASES. doi:10.1021/acsinfecdis.2c00456
Alcott, A. M., Werner, L. M., Baiocco, C. M., Dufrisne, M. B., Columbus, L., & Criss, A. K. (2022). Variable Expression of Opa Proteins by Neisseria gonorrhoeae Influences Bacterial Association and Phagocytic Killing by Human Neutrophils. JOURNAL OF BACTERIOLOGY, 204(4). doi:10.1128/jb.00035-22
Ray, J. C., Smirnov, A., Maurakis, S. A., Harrison, S. A., Ke, E., Chazin, W. J., . . . Criss, A. K. (2022). Adherence Enables Neisseria gonorrhoeae to Overcome Zinc Limitation Imposed by Nutritional Immunity Proteins. INFECTION AND IMMUNITY, 90(3). doi:10.1128/iai.00009-22
2021
Criss, A. K., Genco, C. A., Gray-Owen, S. D., Jerse, A. E., & Seifert, H. S. (2021). Challenges and Controversies Concerning Neisseria gonorrhoeae-Neutrophil Interactions in Pathogenesis. MBIO, 12(3). doi:10.1128/mBio.00721-21
Criss, A. K., Genco, C. A., Gray-Owen, S. D., Jerse, A. E., & Seifert, H. S. (2021). Challenges and Controversies Concerning Neisseria gonorrhoeae-Neutrophil Interactions in Pathogenesis. MBIO, 12(3). doi:10.1128/mBio.00721-21.h-seifert@northwestern.edu
2020
Werner, L. M., Palmer, A., Smirnov, A., Dufrisne, M. B., Columbus, L., & Criss, A. K. (2020). Imaging Flow Cytometry Analysis of CEACAM Binding to Opa-Expressing Neisseria gonorrhoeae. CYTOMETRY PART A, 97(10), 1081-1089. doi:10.1002/cyto.a.24037
Ragland, S. A., Gray, M. C., Melson, E. M., Kendall, M. M., & Criss, A. K. (2020). Effect of Lipidation on the Localization and Activity of a Lysozyme Inhibitor in Neisseria gonorrhoeae. JOURNAL OF BACTERIOLOGY, 202(8). doi:10.1128/JB.00633-19
2019
Smirnov, A., Solga, M. D., Lannigan, J., & Criss, A. K. (2020). Using Imaging Flow Cytometry to Quantify Neutrophil Phagocytosis. NEUTROPHIL: METHODS AND PROTOCOLS, 3RD EDITION, 2087, 127-140. doi:10.1007/978-1-0716-0154-9_10
Maurakis, S., Keller, K., Maxwell, C. N., Pereira, K., Chazin, W. J., Criss, A. K., & Cornelissen, C. N. (2019). The novel interaction between Neisseria gonorrhoeae TdfJ and human S100A7 allows gonococci to subvert host zinc restriction. PLOS PATHOGENS, 15(8). doi:10.1371/journal.ppat.1007937
Allen, L. -A. H., & Criss, A. K. (2019). Cell intrinsic functions of neutrophils and their manipulation by pathogens. CURRENT OPINION IN IMMUNOLOGY, 60, 124-129. doi:10.1016/j.coi.2019.05.004
Kuhn, J., Smirnov, A., Criss, A. K., & Columbus, L. (2019). Quantifying Carcinoembryonic Antigen-like Cell Adhesion Molecule-Targeted Liposome Delivery Using Imaging Flow Cytometry. MOLECULAR PHARMACEUTICS, 16(6), 2354-2363. doi:10.1021/acs.molpharmaceut.8b01274
Ragland, S. A., & Criss, A. K. (2019). Protocols to Interrogate the Interactions Between Neisseria gonorrhoeae and Primary Human Neutrophils. NEISSERIA GONORRHOEAE: METHODS AND PROTOCOLS, 1997, 319-345. doi:10.1007/978-1-4939-9496-0_19
2018
Palmer, A., & Criss, A. K. (2018). Gonococcal Defenses against Antimicrobial Activities of Neutrophils. TRENDS IN MICROBIOLOGY, 26(12), 1022-1034. doi:10.1016/j.tim.2018.07.003
Ragland, S. A., Humbert, M. A. V., Christodoulides, M., & Criss, A. K. (2018). Neisseria gonorrhoeae employs two protein inhibitors to evade killing by human lysozyme. PLOS PATHOGENS, 14(7). doi:10.1371/journal.ppat.1007080
Stevens, J. S., Gray, M. C., Morisseau, C., & Criss, A. K. (2018). Endocervical and Neutrophil Lipoxygenases Coordinate Neutrophil Transepithelial Migration to Neisseria gonorrhoeae. JOURNAL OF INFECTIOUS DISEASES, 218(10), 1663-1674. doi:10.1093/infdis/jiy347
Handing, J. W., Ragland, S. A., Bharathan, U. V., & Criss, A. K. (2018). The MtrCDE Efflux Pump Contributes to Survival of Neisseria gonorrhoeae From Human Neutrophils and Their Antimicrobial Components. FRONTIERS IN MICROBIOLOGY, 9. doi:10.3389/fmicb.2018.02688
Stevens, J. S., & Criss, A. K. (2018). Pathogenesis of Neisseria gonorrhoeae in the female reproductive tract: neutrophilic host response, sustained infection, and clinical sequelae. CURRENT OPINION IN HEMATOLOGY, 25(1), 13-21. doi:10.1097/MOH.0000000000000394
2017
Ragland, S. A., & Criss, A. K. (2017). From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLOS PATHOGENS, 13(9). doi:10.1371/journal.ppat.1006512
Criss, A. K., & Tang, C. (2017). Neisseria: recent advances and future challenges. PATHOGENS AND DISEASE, 75(8). doi:10.1093/femspd/ftx090
Smirnov, A., Solga, M. D., Lannigan, J., & Criss, A. K. (2017). High-Throughput Particle Uptake Analysis by Imaging Flow Cytometry.. Current protocols in cytometry, 80, 11.22.1-11.22.17. doi:10.1002/cpcy.19
2016
Ragland, S. A., Schaub, R. E., Hackett, K. T., Dillard, J. P., & Criss, A. K. (2017). Two lytic transglycosylases in Neisseria gonorrhoeae impart resistance to killing by lysozyme and human neutrophils. CELLULAR MICROBIOLOGY, 19(3). doi:10.1111/cmi.12662
Jean, S., Juneau, R. A., Criss, A. K., & Cornelissen, C. N. (2016). Neisseria gonorrhoeae Evades Calprotectin-Mediated Nutritional Immunity and Survives Neutrophil Extracellular Traps by Production of TdfH. INFECTION AND IMMUNITY, 84(10), 2973-2985. doi:10.1128/IAI.00319-16
Martin, J. N., Ball, L. M., Solomon, T. L., Dewald, A. H., Criss, A. K., & Columbus, L. (2016). Neisserial Opa Protein-CEACAM Interactions: Competition for Receptors as a Means of Bacterial Invasion and Pathogenesis. BIOCHEMISTRY, 55(31), 4286-4294. doi:10.1021/acs.biochem.6b00124
2015
Smirnov, A., Solga, M. D., Lannigan, J., & Criss, A. K. (2015). An improved method for differentiating cell-bound from internalized particles by imaging flow cytometry. JOURNAL OF IMMUNOLOGICAL METHODS, 423, 60-69. doi:10.1016/j.jim.2015.04.028
Handing, J. W., & Criss, A. K. (2015). The lipooligosaccharide-modifying enzyme LptA enhances gonococcal defence against human neutrophils. CELLULAR MICROBIOLOGY, 17(6), 910-921. doi:10.1111/cmi.12411
Juneau, R. A., Stevens, J. S., Apicella, M. A., & Criss, A. K. (2015). A Thermonuclease of Neisseria gonorrhoeae Enhances Bacterial Escape From Killing by Neutrophil Extracellular Traps. JOURNAL OF INFECTIOUS DISEASES, 212(2), 316-324. doi:10.1093/infdis/jiv031
2014
Johnson, M. B., Ball, L. M., Daily, K. P., Martin, J. N., Columbus, L., & Criss, A. K. (2015). Opa plus Neisseria gonorrhoeae exhibits reduced survival in human neutrophils via Src family kinase-mediated bacterial trafficking into mature phagolysosomes. CELLULAR MICROBIOLOGY, 17(5), 648-665. doi:10.1111/cmi.12389
2013
Smirnov, A., Daily, K. P., & Criss, A. K. (2014). Assembly of NADPH Oxidase in Human Neutrophils Is Modulated by the Opacity-Associated Protein Expression State of Neisseria gonorrhoeae. INFECTION AND IMMUNITY, 82(3), 1036-1044. doi:10.1128/IAI.00881-13
Johnson, M. B., & Criss, A. K. (2013). Fluorescence Microscopy Methods for Determining the Viability of Bacteria in Association with Mammalian Cells. JOVE-JOURNAL OF VISUALIZED EXPERIMENTS, (79). doi:10.3791/50729
Stohl, E. A., Dale, E. M., Criss, A. K., & Seifert, H. S. (2013). Neisseria gonorrhoeae Metalloprotease NGO1686 Is Required for Full Piliation, and Piliation Is Required for Resistance to H2O2- and Neutrophil-Mediated Killing. MBIO, 4(4). doi:10.1128/mBio.00399-13
Ball, L. M., & Criss, A. K. (2013). Constitutively Opa-Expressing and Opa-Deficient Neisseria gonorrhoeae Strains Differentially Stimulate and Survive Exposure to Human Neutrophils. JOURNAL OF BACTERIOLOGY, 195(13), 2982-2990. doi:10.1128/JB.00171-13
Johnson, M. B., & Criss, A. K. (2013). Neisseria gonorrhoeae phagosomes delay fusion with primary granules to enhance bacterial survival inside human neutrophils. CELLULAR MICROBIOLOGY, 15(8), 1323-1340. doi:10.1111/cmi.12117
2012
Criss, A. K., & Seifert, H. S. (2012). A bacterial siren song: intimate interactions between Neisseria and neutrophils. NATURE REVIEWS MICROBIOLOGY, 10(3), 178-190. doi:10.1038/nrmicro2713
2011
Johnson, M. B., & Criss, A. K. (2011). Resistance of Neisseria gonorrhoeae to neutrophils. FRONTIERS IN MICROBIOLOGY, 2. doi:10.3389/fmicb.2011.00077
2010
Schook, P. O. P., Stohl, E. A., Criss, A. K., & Seifert, S. (2011). The DNA-binding activity of the Neisseria gonorrhoeae LexA orthologue NG1427 is modulated by oxidation. MOLECULAR MICROBIOLOGY, 79(4), 846-860. doi:10.1111/j.1365-2958.2010.07491.x
Criss, A. K., Bonney, K. M., Chang, R. A., Duffin, P. M., LeCuyer, B. E., & Seifert, H. S. (2010). Mismatch Correction Modulates Mutation Frequency and Pilus Phase and Antigenic Variation in Neisseria gonorrhoeae. JOURNAL OF BACTERIOLOGY, 192(1), 316-325. doi:10.1128/JB.01228-09
